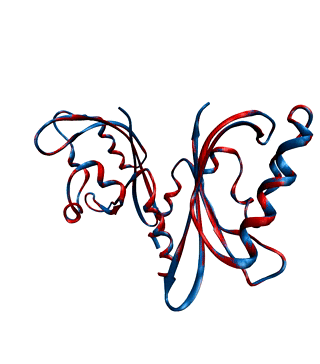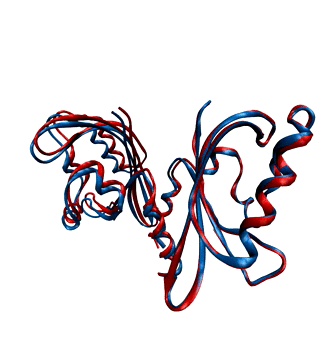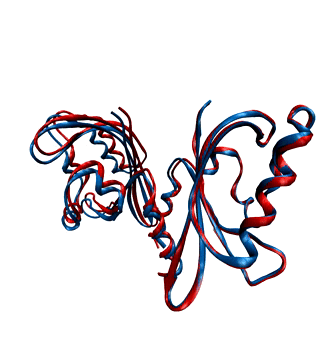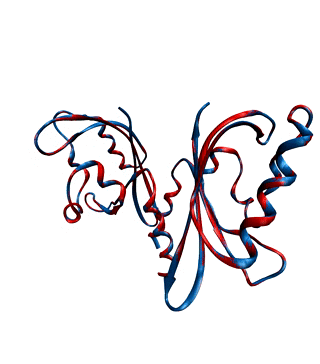Research PROJECTS
PHOTORECEPTOR PROTEINS
Environmental conditions influence the growth, development and regulations of living organisms. One of the most important environmental factors is light. Plants, fungi, and bacteria sense and react to ambient light levels using photoreceptor proteins. Several classes of photoreceptors exist. For instance, phytochromes detect red and far-red light and mediate a wide range of photomorphogenic responses. Cryptochromes and phototropins, two other classes of photoreceptors, absorb UV/blue light.
We want to decipher the molecular workings of photoreceptor proteins using time-resolved X-ray scattering and crystallography. We frequently use synchrotron and X-ray Free Electron Laser facilities world-wide, such as the XFELs in Japan and the US, and synchtrotrons in France, the US, and Germany.
With our national and international collaborations, we have contributed a number of findings towards understanding the structural photoactivation of these proteins (see publications).
CHEMICAL REACTIONS DYNAMICS
The study and understanding of chemical reactions forms the essence of chemistry. Chemical reactivity is critically controlled by the structures of transition states, intermediates, and their solvent-cages. The motion of atoms occurs on a femtosecond time scale. Therefore, the characterization of early reaction events requires ultrafast laser-triggers.
The recent availability of intense, femtosecond, hard X-ray pulses from X-ray Free Electron Lasers (XFELs) enable the visualization of the motion of atoms during the earliest steps of chemical reactions. Our team along with our collaborators have been awarded beamtime at the Japanese XFEL SACLA and the LCLS at Stanford in the United States of America.
We focus on directly visualizing the structural evolution of chemical reactions with time-resolved Wide Angle X-ray Scattering. The data encode the evolution of the distances between atoms on sub-picosecond times, revealing nascent short-lived reaction intermediates, solvent-solute interactions, and their associated dynamics. Ultimately, our goal is to link these various phenomena to the reaction coordinate, providing a detailed, physical understanding of the chemical reaction.
METHOD
TIME-RESOLVED WIDE ANGLE X-RAY SCATTERING
To visualize molecular structural dynamics we use time-resolved X-ray diffraction and time-resolved spectroscopy.
Sebastian developed time-resolved wide-angle x-ray scattering to detect membrane protein structural changes during his PostDoc with Prof Neutze (Andersson et al., Structure 2009; Westenhoff et al., Nature Methods 2010). Time-resolved WAXS acts on proteins in solution, which is the natural environment for most proteins and structural perturbations by crystal contacts are avoided. The technique is particularly suited for detecting rearrangements of secondary, tertiary, or quaternary structural elements.
We have also developed computational approaches to interpret difference WAXS patterns in terms of structural changes. In a molecular dynamics simulation, the proteins are driven to conformations that agree with the difference WAXS patterns (Björling et al., JCTC 2015).
TIME-RESOLVED VIBRATIONAL SPECTROSCOPY
Vibrational spectroscopy is a powerful probe of chemical change. We have a femtosecond infrared spectrometer to measure two-dimensional infrared spectra and infrared spectra in response to an optical pump laser.
In proteins, spectral congestions is a major problem. To overcome this problem, we have developed a chemical method to place isotopes site-selectively in proteins. The isotopes cause a shift of peaks in the spectra, which can then be assigned to specific sites in the protein (see Figure). The method was demonstrated by inserting an isotope-labelled tyrosine into GFP (Peuker et al., JACS 2016).